Six Sigma and its DMAIC (Define, Measure, Analyze, Improve, Control) methodology provide a structured process for solving problems and improving processes. For this project, our team used DMAIC to improve a problem-solving process used in the medical industry – the CAPA process.
Medical device companies are required to demonstrate compliance to the Federal Drug Administration (FDA) 21 Part 820.100 Corrective Action and Preventive Action (CAPA) to be able to sell medical devices in the United States.
The CAPA process at Medtronic complies with the regulations of the FDA and applicable international standards to address quality issues – device complaints, non-conformances and audit findings. The CAPA process is divided into three key phases and align with the DMAIC phases as shown in the table below.
| Relationship Between DMAIC and CAPA Phases | ||
| CAPA Phase | What Happens in Phase | DMAIC Phase Correlation |
| Investigation | Determine root cause | Define, Measure, Analyze |
| Action | Take corrective action | Improve |
| Effectiveness | Verify the success of the corrective action | Control |
Overview
In this project, the team of quality managers and engineers used a DMAIC process to improve the CAPA process. The existing process was complex, leading to several inefficiencies including rework of CAPA tasks and delays in getting the tasks completed on time. The CAPA owners faced several challenges in writing the tasks and needed guidance to complete their work.
The team gathered the voice of the internal customers by:
- Soliciting feedback from CAPA owners (people who were responsible for following the CAPA problem-solving process to resolve a specific problem)
- Performing KJ analysis or affinity diagramming to group the voices by common themes
- Prioritizing sets of customer needs and converting them to measurable requirements
The measurable requirements were flowed down, and concepts were generated using TRIZ (the Theory of Inventive Problem Solving – more on this later) and a concept was selected using a Pugh matrix. Risks were evaluated and the potential favorable or unfavorable impact was statistically modeled using Monte Carlo simulation.
Define
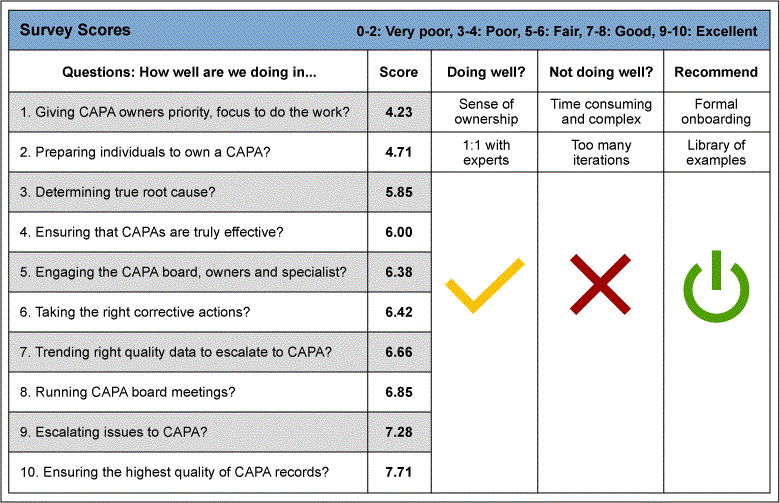
The Define phase began with the gathering of the voice of the customer. The team gave stakeholders of the CAPA process and quality managers a survey shown in Figure 1. The data gathered from this survey gave the team the insight to the customer needs: the CAPA process stakeholders needed guidance and examples to help with writing a new CAPA. This survey was a questionnaire in which individuals were asked to fill out what is going well with the CAPA process, what is not going well and what are the recommendations to improve the process. Each question was rated from 0 to 10, where 0 is the worst score and 10 is the best score.
To gather ideas on the type of platform to use to provide the guidance material to CAPA owners, a KJ analysis (similar to affinity diagramming) was used. This analysis was performed with the stakeholders of the CAPA process, which included CAPA owners and quality managers. The stakeholders brainstormed ideas based on the customer inputs. Ideas were written on sticky notes, organized on a white board and prioritized based on the key customer themes. (Figure 2)
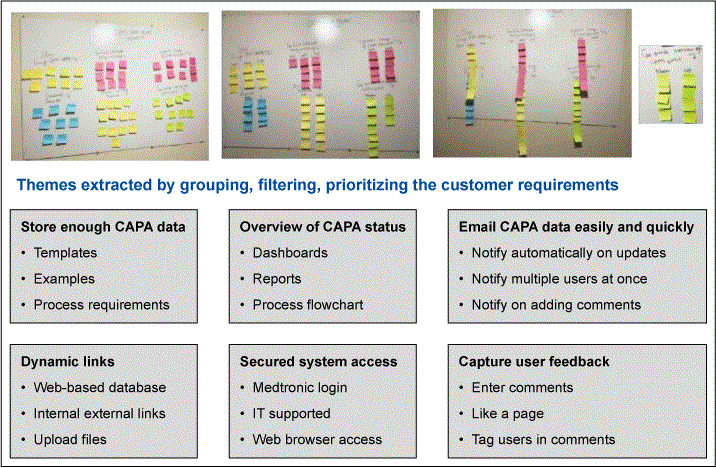
The KJ analysis identified the main theme to improve: Provide CAPA owners with easily accessible examples and templates to help with the CAPA process. Being able to quickly look up the examples and templates that will help the CAPA owners in writing their CAPA tasks.
This proposed solution took the form of a CAPA Portal: a web-based system that provides several templates and examples to complete CAPAs while meeting the compliance requirements and international standards.
Measure
Measurable requirements for the CAPA process are the timely completion with only a few rework cycles associated with completing a CAPA while meeting the FDA’s quality and compliance requirements. The CAPA Portal was developed with these three requirements for measurement:
- CAPA disposition time: The total time to resolve a problem through the CAPA process
- Number of CAPA rework loops: The number of times each step in the CAPA process must be repeated to fix issues
- CAPA resolution time: The time that it takes to resolve a CAPA issue
These key requirements were flowed down from customer expectations using the House of Quality partially shown in Figure 3, and the team prioritized the requirements for the CAPA Portal. The prioritization involved assessing how well each measurable system requirement (left to right) could fulfill each customer requirement (top to bottom). If the system requirement could strongly improve meeting a specific customer requirement, an H for High was entered and assigned a relative value of 9. If there was a medium improvement, an M for Medium and a relative value of 3 was assigned. If there was a low improvement, then an L for Low and a relative value of 1 was assigned. The requirements were then flowed down to sub-system, component and lower-level component requirements.
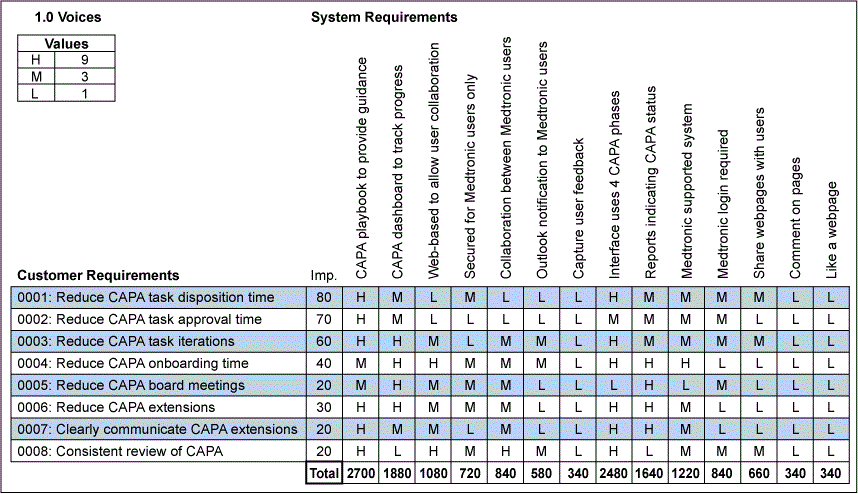
For each column associated with each system requirement, the value of 1, 3 or 9 was multiplied by the relative importance (“Imp”) for that associated customer requirement and summed for the column. This resulted in high priorities for CAPA Portal (aka the “CAPA Playbook”) that would provide guidance along with Interface System Requirements and expectations for a CAPA Dashboard to summarize progress and results.
Analyze
Prior to implementing the requirements, it was necessary to identify the potential sources of failures that can lead to an ineffective CAPA. For the Analyze phase, FDA regulatory expectations and internal business expectations for effectiveness drove the team to understand sources of failure. Fault tree analysis (FTA) was performed to better understand what leads to a deficient CAPA record or an ineffective CAPA. The source of the failure? Deficient CAPA records are due to lack of access to guidance and inadequacies in training. Moreover, the analysis gave the team an insight on the causes that caused poor outcomes, as shown in Figure 4.
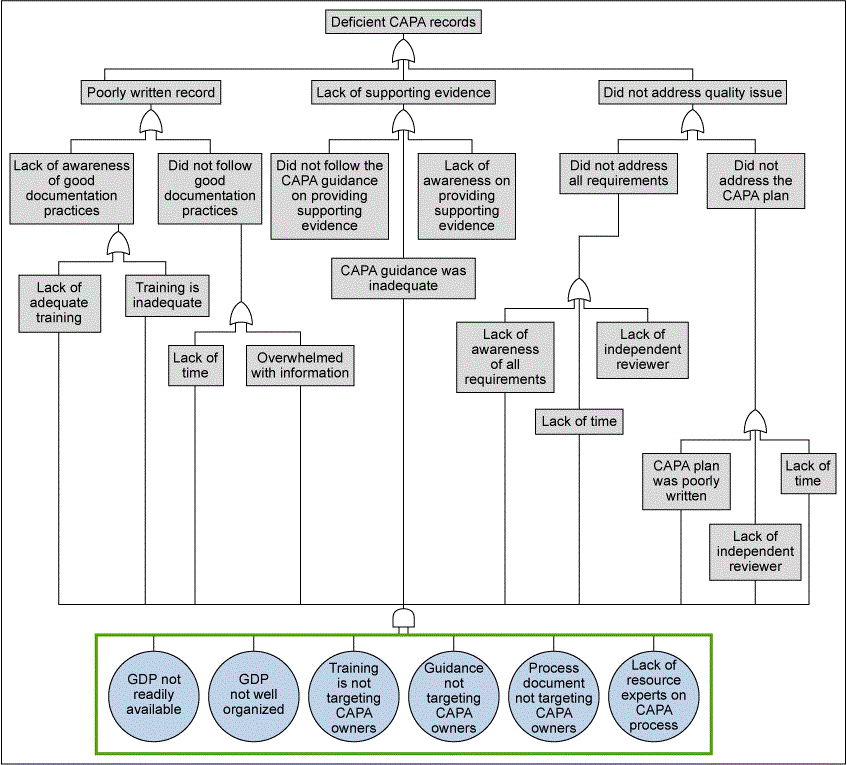
From the FTA, a primary cause of ineffective CAPAs was related to lack to the to-the-point training material. This reinforced the team’s belief that the CAPA Portal must behave as a CAPA Playbook, providing step by step instructions with helpful examples and templates.
The requirement flow-down indicated that the user interface should make it easy for multiple CAPA owners to access and share information. Sharing and communication could enable more rapid resolution of the CAPA issues.
Developing a user interface involves tradeoffs. The TRIZ (Theory of Inventive Problem Solving) concept-generation approach provided a way for the team to dispassionately consider the tradeoffs and find an innovative solution to meet expectations involved in the tradeoff. The TRIZ approach converts tradeoffs into generic tradeoffs and recommends a small set of TRIZ principles that have been used to resolve that sort of generic tradeoff in the past, based on engineer and inventor Genrich Altshuller’s research of millions of patents. Our team used TRIZ to find solutions for the following tradeoffs.
Tradeoff 1:
-
- Feature to improve: Report out on CAPA metrics to increase productivity by 25 percent
- Undesired result: The user could be too overwhelmed by content to consume information
Tradeoff 2:
-
- Feature to improve: Provide a workspace for CAPA owners to fill out the templates
- Undesired result: Inability to download file due to slow speed
Tradeoff 3:
-
- Feature to improve: Make content available with templates and examples on a dedicated page
- Undesired result: Site unable to load all the content
From here, we identified three TRIZ principles (aka known solutions), which were applied to the CAPA Portal to address the aforementioned tradeoffs. The three principles applied to the CAPA Playbook were:
- Principle of Universality: Allows a part of the system to perform multiple functions so other parts can be eliminated. This principle was applied to create dashboards and organize information to report out on CAPA metrics such number of open and closed CAPAs, CAPA age, etc. This solution addressed Tradeoff 1 to solve adaptability versus productivity of the CAPA Portal.
- Principle of Preliminary Action: Allows pre-arranging the elements of the system so that they perform rapidly. This principle was used to attach notes to guide the user and files that serve as a CAPA template that users can access directly from the system. This solution addressed Tradeoff 2 to solve speed versus extent of automation of the CAPA Playbook.
- Principle of Segmentation: Allows separating an element of a system into smaller interconnected elements. This principle was used to provide dedicated links on the CAPA Portal Interface to access the three key phases of the CAPA process: investigation, action and effectiveness. This solution addressed Tradeoff 3 to solve productivity versus reliability of the CAPA Playbook.
Using these TRIZ principles, the team was able to design the system interface and dashboard for the CAPA Portal.
Improve
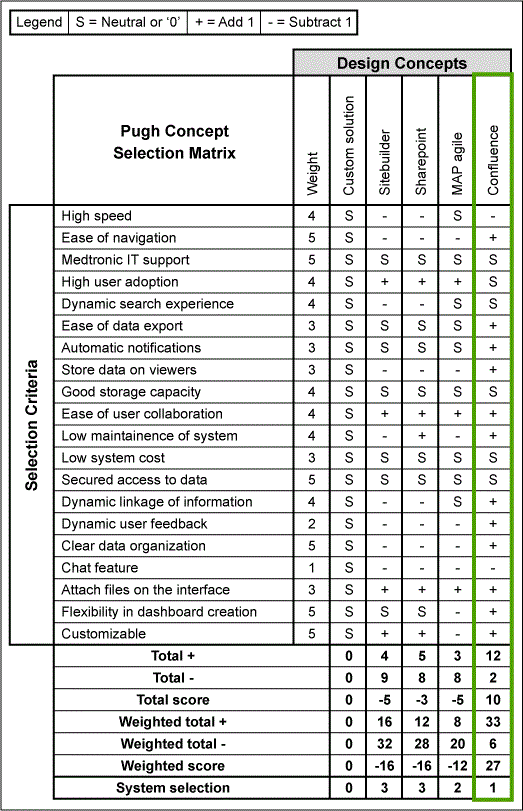
Based on the user criteria for the CAPA Portal established in the Define and Measure Phases, a Pugh matrix was used to evaluate the strengths and weaknesses of the available systems – Sitebuilder, SharePoint, MAP AGILE and Confluence – and rated using S = Neutral or 0, + = add 1, and – = subtract 1 for each selection (Figure 5). The total score and the weighted total were then calculated to identify the system that had the highest score. The CAPA Portal was developed using a web-based system that can be shared with multiple users and can be used to easily access guidance material such as templates and examples.
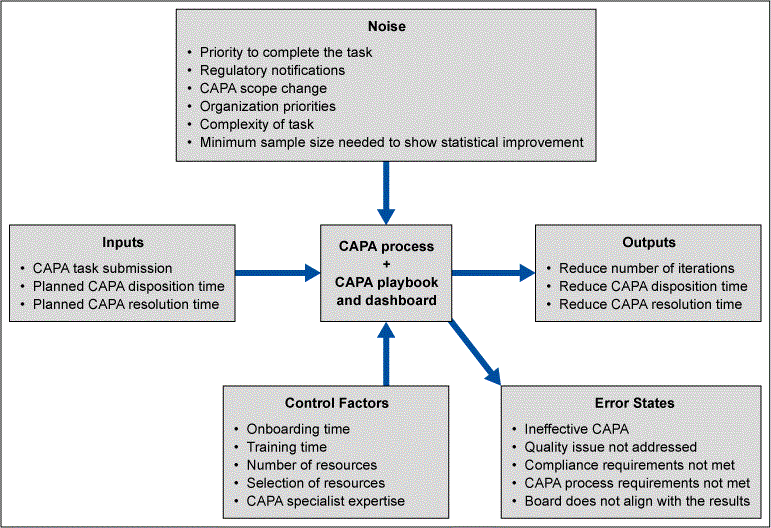
To ensure that the proposed CAPA Portal would meet the users’ expectations over a range of use conditions or noise factors, a P-diagram (parameter diagram) was used (Figure 6). It showed the interactions of the system, the inputs and outputs, the noise factors, control factors and error states. Error states from the P-diagram were evaluated further as failure modes through failure mode and effects analysis (FMEA). The FMEA helped the team analyze risks, prioritize risks and take actions to mitigate the risks. From this FMEA, the CAPA Portal was designed to anticipate potential error states and provide early warnings and direct users to mitigation through help options.
Through implementation in the Improve phase of DMAIC, users of the CAPA Portal began accessing the new CAPA Playbook to guide them in their CAPA tasks. The value of this system was measured using the critical parameters of disposition time, rework and resolution time for each CAPA task.
The results from critical parameters measured over a period of May 1, 2019, to January 11, 2020 (before the deployment of the CAPA Portal), and January 12, 2020, to July 7, 2020 (after the deployment of the CAPA Portal).
- The number of rework loops in approving a CAPA task decreased by 61 percent.
- The total time in review and approval of a CAPA task decreased by 53 percent.
- The total time to resolve a rejection of a CAPA task decreased by 43 percent.
Control
The transition from the Improve phase to the Control phase of DMAIC typically includes overcoming resistance to change, implementation of control mechanisms such control charting, mistake proofing (poka yoke) and institutionalization.
Since the users and other stakeholders were engaged throughout this project, from gathering their own voices through being involved in generating and selecting concepts, there was little resistance to overcome. Rather, users were extremely receptive.
The control mechanism was provided by the CAPA Dashboard that was integrated into the CAPA Portal. Poka yoke was integrated into the user interfaces and help systems. The CAPA Playbook was institutionalized within the original organization through documented and controlled processes; it is being used for all CAPAs, with its immediate feedback and control.
The results were rapidly shared with executives including vice presidents of quality and manufacturing operations. The executives were impressed by the project’s impressive results, and the executives requested that the CAPA Playbook and associated improvements to the CAPA process be replicated through other parts of the organization.