
As is true throughout virtually all areas of business across diverse industry sectors, Lean Six Sigma principles and practices applied to research and development (R&D) and product development in the pharmaceutical and biotechnology industries have a dramatic effect on reducing cycle times and the cost of developing new technologies, products, and protocols for research and clinical applications.
Although pharma and biotech have only recently begun to adopt and implement Lean Six Sigma, their potential is already being realized. Companies working to integrate the tools and culture of Lean Six Sigma into their infrastructure and operations are quickly recognizing the gains that have historically been well documented in other industries. These include higher throughputs, increased capacity and productivity, fewer errors, and better utilization of staff, facilities and resources.
With steadily increasing R&D costs, lagging productivity, and the continuing need to adopt, implement, and integrate novel processes and technologies, the pharma and biotech sectors are well-positioned to benefit from the advantages that Lean Six Sigma has to offer. Its robust, statistically sound methods and tools will help these industries conserve resources, optimize protocols and processes, and streamline operations. This structured approach complements the U.S. Food and Drug Administration’s process analytical technology initiative published in 2003 to design quality into drug products, rather than test quality into final dosage forms. Over time, Lean Six Sigma will become a seamless and critical operational strategy that will enable lasting change and significant improvements in efficiency and productivity.
Lean and Six Sigma are complementary strategies; when implemented individually, they each offer significant benefits. But when applied together in a cohesive, integrated approach, their synergies yield further improvements in throughput, reproducibility and quality. Both Lean and Six Sigma are customer-focused, data-based and results-driven processes that support decision making and optimize outcomes.
Targeting Drug Discovery and Development
The pharmaceutical industry will have to increase substantially the productivity of its R&D efforts to return to the double-digit growth rates it enjoyed in years past. Since the 1970s, the number of new drugs introduced each year has steadily declined, while the cost to develop a new drug (now $880 million on average) and the time to market (15 years) have steadily increased. About 90 percent of all drugs in development will fail to come to market, and many of those will fail in late-stage development, when significant resources have already been invested to move them toward human trials.
| Common Misperceptions About Lean Six Sigma and Pharma/Biotech | |
| What Some People Say | Why Lean Six Sigma Works for Pharma/Biotech |
| “We’re different! We don’t make cars or aircraft engines.” | Pharma/Biotech’s products are new molecules, reagents or instruments. Lean Six Sigma principles can be applied to these continuous (as opposed to discrete) processes. |
| “R&D is not a process.” | The output of R&D is knowledge. The flow of knowledge can be tracked as if it were a physical artifact. |
| FDA/EMEA regulations do not allow implementation of Lean Six Sigma.” | Data-based approach of Lean Six Sigma complements FDA’s process analytical technology initiative. Also, Lean Six Sigma has been successfully implemented in other regulated industries – for example, the aviation industry that is regulated by the Federal Aviation Administration. |
| “Our industry has long product cycle times.” | Long processes can be subdivided into sub-processes and analyzed. |
| “Product experimentation is expensive.” | Lean Six Sigma incorporates several statistical techniques to reduce the number of experiments and prototypes. |
To hasten the discovery of innovative therapeutics and improve the economics and efficiency of pharmaceutical R&D the industry must identify novel high-throughput technologies, streamline existing processes and, perhaps most importantly, develop methods – experimental and computational – that will enable it to identify as early as possible in the R&D pipeline those drug candidates that are destined to fail in preclinical and clinical testing. Lean Six Sigma can help companies maximize the output of their R&D operations, shorten product development cycles and drive innovation. The end result will be greater productivity.
Using Lean to Reduce Cycle Times
Lean drives cycle time reduction, enabling product development with minimal resource utilization. Its aim is to get the job done better and faster – in essence, to create the perfect process. It achieves this by optimizing processes and eliminating waste. In this context “waste” refers to any type of excess, including tangible waste, such as excess inventory, defective materials, equipment malfunctions or production errors, and intangible losses, such as time spent waiting on the job, delayed delivery to customers, or equipment down-time due to mechanical problems or disuse. Lean identifies and targets waste in the process stream, thereby increasing capacity, improving quality and maximizing efficiency.
The primary objectives of Lean are to identify the sub-processes that either add or do not add value to the final objectives, to see where there is waste, and to minimize or eliminate non-value-added factors. This is achieved by mapping the value stream, prioritizing improvement opportunities, and applying modeling and analytical tools to implement and assess improvements. The goal is to gain a better understanding of current processes, identify and anticipate needs, and develop a plan for change.
Using Six Sigma to Reduce Defects
Six Sigma offers particular advantages for drug discovery and development. Biology is inherently a gray science, and biological systems are fraught with variation. Consider, as an example, functional assays intended to assess the effects of a stimulus on live cells. When repeated multiple times using the same type and number of cells and performed under the same conditions, these assays will not yield identical results in terms of how the cells respond to a particular stimulus. This is due to the variation intrinsic to living systems.
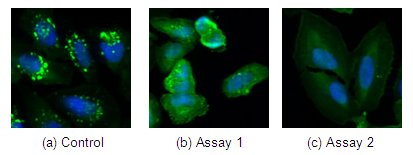
In the photographs above, cells are labeled with green fluorescent protein (GFP), which localizes to the endosomes (green dots in control). Upon treatment with an inhibitor, endosomal localization is lost and GFP localizes uniformly throughout the cytoplasm. Three parameters can be used to quantify this effect – the number, size and total fluorescence of the granules. As shown, not all cells respond equally or completely to the treatment. For example, the control (a) shows an average of 14 granules per cell. The same kind of cells after treatment can show a wide range of response – (b) shows about five granules versus none in (c). Many biological systems show large variation in responses.
Ideally, the assay should perform reliably and reproducibly despite variation in the system. When applying Six Sigma, two types of variation must be considered – imperfections in the product (the assay) itself, and variation in how the product is used. These should be taken into account upfront, during design and development of the product.
Even minor changes in input and product usage can cause large variation in output, and this variation can be minimized through an understanding of the potential spectrum of variation, analysis of its possible effects on the system and on outcomes, and thoughtful product design. The goal is to design a process or product such that the likelihood it will be used correctly (within the range in which it was designed to function accurately – its “sweet spot”) is maximized, and the variation in input and usage that does occur will case the least possible variation in output.
Case Study: Applying Design for Six Sigma
Lean Six Sigma is an intrinsic component of the language and culture at GE, and it has had a significant impact on operational efficiency, productivity and the quality of products and services provided to customers. In addition to internal process improvements achieved through Lean Six Sigma, GE has achieved a competitive edge by applying Design for Six Sigma (DFSS) to product development. DFSS tools and techniques are data-driven methods for systematically and statistically assessing factors related to product efficacy and safety and quantifying probable outcomes using computer models and simulations based on system physics. DFSS enables a proactive, rather than reactive, approach to design that takes into consideration upfront customer expectations, critical design elements, and predictions of quality and robustness.
In the pharma/biotech sector, DFSS was applied to the development of a method for amplifying nanogram starting amounts of DNA to generate microgram quantities for experimental use. Two teams of scientists participated in the project, aimed at optimizing the reagent formulations: one employed a traditional approach to reagent development, conducting sequential experiments to optimize one factor at a time; while the second team used a multifactorial approach, applying DOE (design of experiments – a structured method for determining the relationship between factors affecting a process and the output of that process) to optimize the formulation by identifying the reagent’s sweet spot and making it robust to variation.
Keys to Lean Six Sigma Success
- Need strong support from top leadership
- Assign best people to solve the tough problems
- Link Lean Six Sigma activities to company’s metrics of corporate success
- Focus on end results and avoid over-emphasis on use of tools
- Communicate clearly and often to all levels of the organization
The results were dramatic:
- Reduced cycle (product development) time from three weeks to three days with DFSS compared to the conventional approach.
- Design determined following only two sets of experiments (versus seven).
- Lower overall development cost and resource utilization.
- A better product – a reagent that works faster and more efficiently and is robust to variation in manufacturing and customer use.
Conclusion: Targeting Waste and Variation
By applying Lean Six Sigma tools and techniques to drug discovery R&D, the pharma and biotech industries can eliminate waste and minimize variation in process streams, improving the quality, reliability and reproducibility of outcomes. These improvements will be propagated and perhaps even amplified upstream and downstream of the drug discovery and development pipeline, generating significant gains in operational efficiency, resource conservation, and overall productivity. These gains will be realized across all sectors of the internal R&D effort and will positively impact interactions with end-users, such as regulatory agencies, customers and commercial collaborators.